Design References
mAb Production Facility, Eastern Europe
Project Details
Project Details
Client
Generium
Location
Eastern Europe
Market
Biologics
Solutions we provided
Design based on MBS design platform
square meters workshop space
Certified 14001
Project Overview
Project Overview
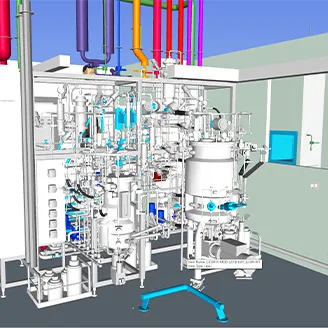
When a biotech company best known for specialising in rare diseases and for producing “orphan” pharmaceuticals needed a new production facility in eastern Europe, it asked KeyPlants to develop layouts, process flow diagrams, and more.
The solution for the greenfield 4080-sqm mAbs and therapeutic proteins production facility is based on our unique MBS (Modular Biotech Solutions) platform. One of the challenges was the need to have two completely separate production lines for upstream and downstream of mAbs and therapeutic proteins.
“They chose us partly because of our solution and our experience in design, engineering, and regulatory issues,” recalls Tanja Sjödin, Regional Manager. “Their previous experience of working with KeyPlants on other projects was also a factor.”
The overall size of the facility is 4080 sqm with a 2060-sqm processing area.